|
Quick
Links: The
Rocks,
The Soil, Branchville
Mine, Redding Garnets
The
Rocks:
The geological character of the town was described as metamorphic
by the Rev. John Dickinson. Granite, porphyritic rock and
especially micaceous schist pre-dominated minerals as are
familiar in such rock as hornblende, garnet, kyanite and tremolite.
One needs not travel far to find Redding's rock outcroppings.
Out the backdoor of most any household you will find the "chiseled"
results of natures splendor. The most dramatic geologic masses
can be seen along the Falls Trail in the Saugatuck River Nature
Reserve, the Great Ledge in Devil's Den, and on "Indian's
Lookout" in The Rock Lot/Scott's Preserve.
On a recent walk
in Devil's Den, local historian Roy Spies explained that the
Wisconsin III glacer halted in this area, and in melting dropped
much of the rock we see here today; existing bedrock was scrapped
of its soil cover as the glacer moved across the land. He
also mentioned that much of the rich soil within the glacer
was washed out to Long Island providing that region with near
perfect soil for planting.
Magnesian limestone
could be found in the western part of town and was said to
be quite pure. The mining of limestone in town took place
in and around Limkiln and Lonetown. Just outside of town off
Route 53 in Bethel, CT a limestone quarry lives on today as
a swimming hole named fittingly "The Quarry". A
rather famous mine can be found on Mountain Road. Here is
a bit of its history:
Branchville
Mica Mine
The
"World Famous" Branchville Mica mine lies in the town of Redding
on Mountain Road, 550 ft. N.E. of the Branchville railroad
station.
The
first excavation in the Branchville Mine was made about 1876
by Abijiha N. Fillow, then owner of the property. Fillow was
mining for mica. The mica recovered was then considered of
inferior quality, and operations ceased sometime before the
spring of 1878. At that time, George J. Brush and Edward S.
Dana, both of Yale University, became so enthused about the
new minerals at Branchville that they engaged Fillow to excavate
the deposit with funds furnished by Yale.
The
results of these excavations gained the mine worldwide fame
as (9) rare minerals (eight were discovered for the first
time in the history of science) were mined at this unique
location:
(A)
Lithiophilite, maganese-iron phosphate, its name indicates
its lithium content.
(B) Natrophilite, sodium-manganese phosphate, its name indicates
a high sodium content.
(C) Dickinsonite, hydrated acid phosphate of sodium and manganese.
Named in honor of Rev. Dickinson, formerly of Redding, CT.
(D) Fillowite, a hydrated acid phosphate of sodium, manganese,
iron and calcium. Named in honor of A.N. Fillow of Branchville,
CT
(E) Fairfieldite, a hydrated phosphate of calcium. Named in
honor of Fairfield, CT
(F) Eosphorite, a hydrated basis phosphate of aluminum with
iron and manganese. Named from the Greek in allusion to its
pink color.
(G) Reddingite, hydrated phosphate of manganese and iron.
Named in honor of Redding township.
(H) Tripoidite, basis phosphate of manganese and iron. Named
in allusion to its resemblance to triplite in physical character
and composition.
Information
regarding these minerals appeared in scientific journals from
1878 to 1890 launching Brush and Dana's careers and one would
hope- Fillow's real estate property value.

The World
Famous Branchville Mica Mine Today (2006)
In
1880 the Union Porcelain Works of Greenpoint, New York, bought
the property from Fillow and operated it for feldspar and
quartz until 1891. The principal use for feldspar was in the
ceramic industry. Other uses included enameling for metal,
glazes, and abrasives in soaps. At this time the mine was
renamed "The Smith Mine". Fillow stayed on as supervisor of
mining operations but resigned one year later. As a stipulation
in the sale, all unused minerals were to be placed at the
disposal of Brush and Dana.
Next
the Bridgeport Wood Finishing
Company of Bridgeport & later New Milford, Connecticut,
operated for quartz and feldspar at the mine as well as other
locations in the Branchville area from 1891 to 1917. These
"other" areas included Mountain Rd., Pine Mountain Rd. and
parts of the land we now refer to as the Scott Preserve/Rock
Lot. Deeds indicate Jesse Fillow leased a 3-acre triangular
piece to the BWFC at 32 Mountain Rd. in 1911, BWFC transferred
it to Gininone Di Giavanni in 1914. BWFC also leased a 4-acre
tract from John Barrett in 1911 at 34 Mountain Rd. for "the
purpose of searching for quartz or silica; of conducting mining
and quarrying operations and of recovering from here any quartz
or silica…" Details also note "BWFC has the right to renew
this agreement on the same terms and conditions for a further
period of 10 years upon written notice..." the mining operation
called for the removal of 100 gross tons of quartz or silica.
More specifically "if 100 gross tons of quartz or silica are
not mined or quarried, as now contemplated by said parties
within any year during the continuance of this agreement…then
these presents and everything contained therein shall cease
and be forever null and void."

"Silex"
was the trade name for quartz sand, a form of silica BWFC
used extensively in making paste wood fillers; it is chemically
inert, does not absorb moisture or shrink and can be stained
to match any finish. Unfortunately for its workers it was
also extremely damaging to the lungs. BWFC was in business
from 1876 to 1917 when it was purchased by DuPont.

In
addition to Bridgeport Wood Finishing Co. the properties in
and around the mine were leased to several other individuals
and companies in this time frame. For example, a June of 1897
lease between BWFC and William Haaker for 65 acres of land
in Redding and Ridgefield, provided BWFC the right to mine
for quartz and feldspar, and specifically stated that Haaker
only had the right to quarry granite. In 1907 Haaker leased
the same parcel to Traylor Manufacturing and Mining Co. of
New Jersey for the purpose of mining quartz and feldspar for
a period of 5 years. In 1914, Anna Haaker leased the same
parcel to Monarch Mining Co, formerly Traylor Manufacturing
and Mining Company. Traylor Manufacturing and Mining Company
incorporated in 1907 with capital stock of $20,000 and one
year later would increase that stock to $50,000 and change
their name to Monarch Mining Company.
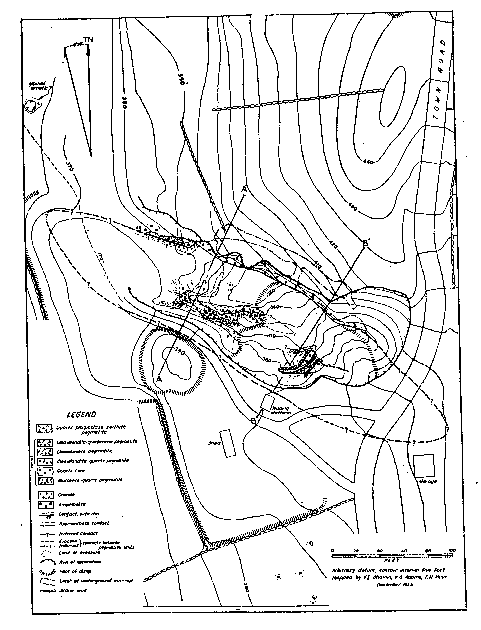
Branchville
Mine. Town Road shown to the right is Mountain Road.
Pine Mountain Road now runs to the north of the mine today.
*Sure wish I had a larger version of this map but at this
point this is all that's available.
J.
Frank Schairer located 31 different minerals at the mine in
1926. It was part of his research work on "The Minerals of
Connecticut." He collected the data while he was at Yale.
From
September 1943 to November 1944, Fred and Joseph Burrone and
Carlo Rusconi, all of North Branford, Connecticut, operated
the mine for mica, and the Sandy Ridge Mica and Mining Company,
Inc., 927 15th Street N. W., Washington, D. C., worked the
mine in November and December 1944. Also in 1944, detailed
studies of the geology were made as part of the strategic-minerals
investigations of the United States Geological Survey.
Sheet
and scrap were the two types of mica mined. Sheet mica was
used primarily for insulating electrical equipment. Specifically
it was used in spark plugs, lamp sockets, radio apparatus,
fuse boxes, heating devices and telephones.
Scrap
mica was used for roofing, wallpaper, paints, for filler in
rubber such as automobile tires, and lubricants. The demand
for sheet mica during World War II induced operators to work
the long dormant mine in 1943 and 1944.
After1944
the mine was sporadically operated until 1954.
The
last attempt to reopen the mine was made in 1979 by geologist,
Michael DeLuca but his request was turned down by the zoning
commission.
About
Mica:
The
word "mica" is thought to be derived from the Latin word micare,
meaning to shine, in reference to the brilliant appearance
of this mineral (especially when in small scales).
Mica
is found abundantly throughout Asia, Africa, as well as North
and South America. Until the 19th century, mica was quite
rare and expensive as a result of the limited supply in Europe.
However, its price dramatically dropped when large reserves
were found and mined in Africa and South America after the
early 19th century.
Mica
has a high dielectric strength and excellent chemical stability,
making it a favored material for manufacturing capacitors
for radio frequency applications. It has also been used as
an insulator in high voltage electrical equipment.
Specific
varieties of mica include:
- Biotite
- Muscovite
- Lepidolite
- Phlogopite
Mica is a general
term for a large group of minerals, hydrous silicates of aluminum
and potassium, often containing magnesium, ferrous iron, ferric
iron, sodium, and lithium and more rarely containing barium,
chromium, and fluorine. All crystallize in the monoclinic
system, but mica is most commonly found in the form of scales
and sheets. All the micas have an excellent basal cleavage,
splitting into very thin, elastic laminae. Some varieties
are transparent; resistance to heat is high.
Commercially, the
most important micas are muscovite (potassium mica) and phlogopite
(magnesium mica).
Muscovite, the
commoner variety, is usually colorless, but it may be red,
yellow, green, brown, or gray, with a vitreous to pearly luster.
It occurs in granites, syenites, mica schists, and gneisses,
but is most common in pegmatite dikes. It is widely distributed.
Phlogopite varies
in color from yellow to brown, some specimens having a coppery
tint and others being greenish. It occurs in crystalline limestones,
dolomites, and serpentines in Canada, New York, New Jersey,
and Finland.
Mica mining, because
of the necessity of keeping the crystals intact, is a delicate
operation; drills and blasting powder must be used carefully,
if at all. The mined crystals are first “cobbed,” i.e., roughly
trimmed of rock and cut, then split with a hammer into plates,
and further split into sheets with a knife.
Sheet mica is used
as an insulating material and as a resonant diaphragm in certain
acoustical devices.
Scrap and ground
mica is used in wallpaper, fancy paint, ornamental tile, roofing,
lubricating oil, and Christmas-tree snow.
Ground mica is
sometimes pressed into sheets (micanite) that can be used
as sheet mica.
Most of the sheet
mica used in the United States is imported, chiefly from India
and also from Brazil. Synthetic mica was produced in the United
States after intensive government-sponsored research began
in 1946.
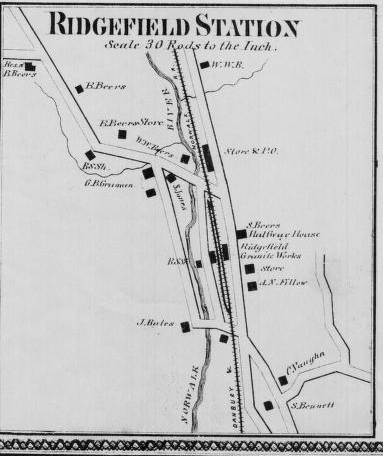
Ridgefield/Branchville
Station in the 1850's
Granite
Mining in Branchville:
Abijiha
N. Fillow's Branchville Mica Mine was by-far the "magnetic
force" that attracted mining companies and geologists to the
area. However, as the map above shows 20 years prior to the
discoveries at Fillow's mine, Philo Bates' Ridgefield Granite
Works was operating out of the station area. In land records
regarding properties on Mountain Rd., Philo W. Bates is listed
in 1875 as owning land "East" of 32 Mountain Rd. and in 1890
as conveying a 20 acre parcel to Abijiha Fillow at 34 Mountain
Rd. which would place at least a portion of his mining areas
on Mountain Rd. and in the areas of the Scott Preserve/Rock
Lot.
Little
is known about the Ridgefield Granite Works aside from the
1856 Clark's Map reference and land records of properties
owned by Philo W. Bates but it is presumed that it was a successful
business given the span of time it operated in the area. Also
appearing on the 1856 station map as a business is Walter
Bates, mason and builder, which very likely involved a joint-venture
with the Granite Works.
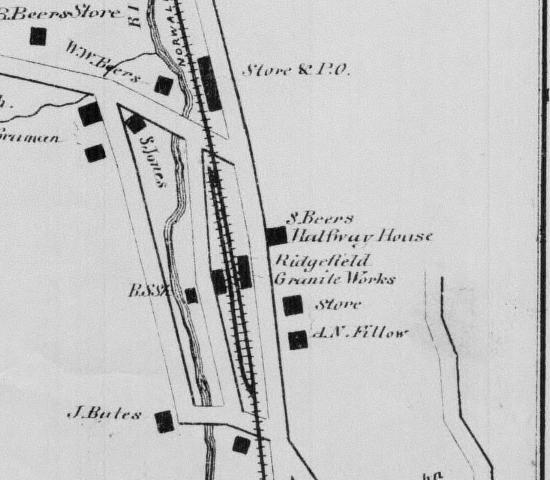
Fillow's
home or business shown in the 1850's down on what is now West
Branchville Road. This map is a close up of Branchville Station
(branchline would come in 1870). Road running behind A.N.
Fillow is Mountain Road. The business next to Fillow is of
interest, Ridgefield Granite Works, location of busines may
shed some light on who was responsible for the
mining evidence up in the Scott's Preserve/Rock Lot.
About Granite
Granite is the
name used for a variety of light-colored, coarse-grained igneous
rocks. Orthoclase (potassium) feldspar is typically the most
abundant mineral in granite and significant amounts of quartz
and plagioclase feldspar are generally present as well. Minor
minerals include muscovite mica, biotite mica, hornblende
and others.

The coarse grain
size of granite indicates a slow rate of cooling that occurred
below the earth's surface. The insulating effect of the surrounding
rock caused the magma to crystallize very slowly. The slow
cooling allowed the mineral grains adequate time to grow to
a large size.

Because it crystallizes
"at depth" Granite exposed at the surface indicates a location
where deep erosion has taken place.
Granite is a very
strong, durable stone and is used in a variety of ways. Its
attractive appearance makes it useful as an architectural
stone. It is also widely used in monuments, grave markers,
stair treads, counter tops, window sills, street curbing and
other dimension stone uses.
Granite is also
used in the form of crushed stone or aggregate. Granite aggregate
is mainly used for road construction and maintenance, however
there are many other uses which include concrete, landscaping
stone and paving. The U.S. Geological Survey estimates that
granite accounts for about 16% of the United States crushed
stone production, behind limestone and dolomite.
What Created
The Mining Scars Found on Granite Rocks in and around Branchville?
Hand drilling was
the method used by quarry miners to extract granite blocks
from the landscape. Hand drilling helps remove rock three
ways: (1) A rock may be split into chunks of manageable size
by steel drilled into a natural seam; (2) If the steel in
the seam does not split the rock by itself, the hole may be
fitted with the wedge and feathers. The wedge is driven between
the feathers with a hammer until the rock breaks; (3) Finally
a hole may be used to prepare a rock for blasting. In general,
the larger the rocks, the more likely you will use explosives
to move them. Although hand drilling was slow work, it was
a safe and simple way to chisel out granite blocks and/or
prepare the rocks for blasting.

Old drill holes
are now filled with moss (middle of photo along crack)
The driller drives
the steel by methodical hammering and turning. When the hammer
strikes the head of the steel, the bit is forced against the
rock. After each blow of the hammer, the driller turns the
steel slightly and strikes it again. With each blow the bit
chips small amounts of rock that collect in the hole as "drilling
dust." The driller removes the dust by adding water to the
hole, which creates a mud that sticks to the sides of the
steel. To clear the mud, the driller removes the steel and
raps it against the rock. The procedure is continued until
the hole is deep enough; longer steel is substituted as the
hole lengthens.

Lone drill hole
with another one started to the left of it.
The steel is manipulated
with one hand while the other hand hammers (single jacking),
or the steel is manipulated by two hands while another person
hammers (double jacking).
Ambidexterity was
very helpful for the single jack driller because he could
work longer by shifting the hammer from one hand to the other
to distribute the work. In double jacking one or two drillers
hit a drilling steel with large sledge hammers while a holder
turned the steel slightly after each blow. As the hole deepened,
the holder substituted longer steels in a way that did not
interrupt the driller's disciplined rhythm.
Redding
Garnets from "The Rock Hound" Redding Times,
1955
Some of the finest
garnets in Connecitcut came from a garnet vein in Redding.
A Mr. Lloyd writing to the editor of the Amercian Journal
of Science dated Weston Conn. May 27, 1820 says: "Garnet
Rock or the precipice in and about which Garnets are found
in abundance and perfection is situated one mile and a half
south of the Congregational Meeting house in the Town of Redding
and about one half mile west of the junction of the two large
branches of the Saugatuck River, which unite a short distance
above the south boundary of the Town of Redding. The northwest
branch of the Saugatuck River runs a winding course in a southerly
direction at the foot of the hill on the top of which are
the rocks under consideration. On top of the hill and at the
base of these rocks on the south east side the Garnets will
be seen by the most inattentive observer, projecting from
the rocks in a manner resembling musket balls shot halfway
into a board."
These large red
garnet crystals are not as easy to find today as they were
in 1820. This is due in part to man's development of the river
areas in this vicinity and the destruction of some of the
better collecting grounds.
Fine cinnamon brown
garnet crystals are also found in Redding and could be seen
in museums and private mineral collections all over the world.
The various well-colored,
transparent garnets are cut as gem stones, the rough granets
are used as an abrasive.
Ronald E. Januzzi
The
Soil:
The Redding area
experienced glaciation in recent geologic time(1.5 million
years ago) the last glacier believed to have receded 10 to
15 thousand years ago according to Former Wetlands Officer
for the Conservation Commission, Stanley Schleifer.
New England soils are very close in composition to their parent
rock. This is due largely to the fact that weather and vegetation
has not had adequate time to breakdown the minerals as it
has in unglaciated regions. Minerals are as prevalent in the
soil as they are in the bedrock that has yielded it. The topsoil
layer that exists averages a depth of 2 1/2" to 8",
a depth that would be suitable for agriculture if not for
the boulders and larger rocks scattered through it. Some areas
contain a sub-soil that makes cultivation possible. This topsoil
mixes with approximately 10 centimeters of sub-soil
and gives the appearance of a thicker soil than actually exists.
Soil is generally thought of as a mixture of particles of
three different sizes: sand, silt, and clay. In New England,
however, we cannot overlook the particles larger than sand
size, such as pebbles, stones, and cobbles. Although these
larger particles do not contribute very much to the physical
and chemical properties of the soil, they do play a crucial
role in determining whether or not the soil is fit for agriculture,
no matter how suitable it is otherwise.
When sand, silt, and clay are present in about equal amounts,
the soil is known as loam. Usually, one or another predominates
and the soil is then further classified as sandy loam, silt
loam, or clay loam.
Southern New England is now about 60 to 75% woodland. Most
of the soils are too sandy, stony, or wet for cultivation.
In fact, probably less than 15% of the area now in woodland
is potential cropland.
Soil maps of most of southern New England resemble intricately
sewn patchwork quilts, showing soils often of widely differing
properties existing in close proximity. Such a condition makes
modern farming almost impossible.
Click below
to continue on reading about the landscape:
Farms
Bodies of Water
Geologic
Back
to TOP | Back to Redding
Section | Back to Georgetown
Section
|

